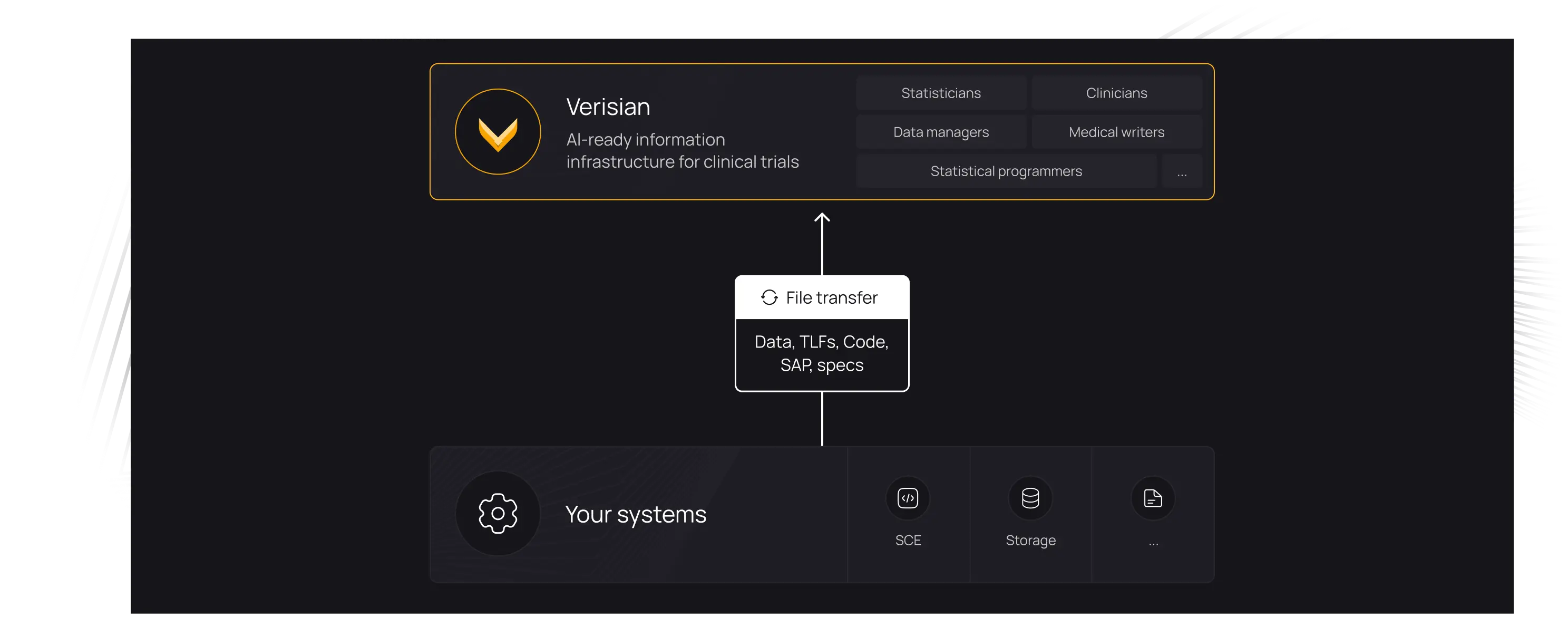
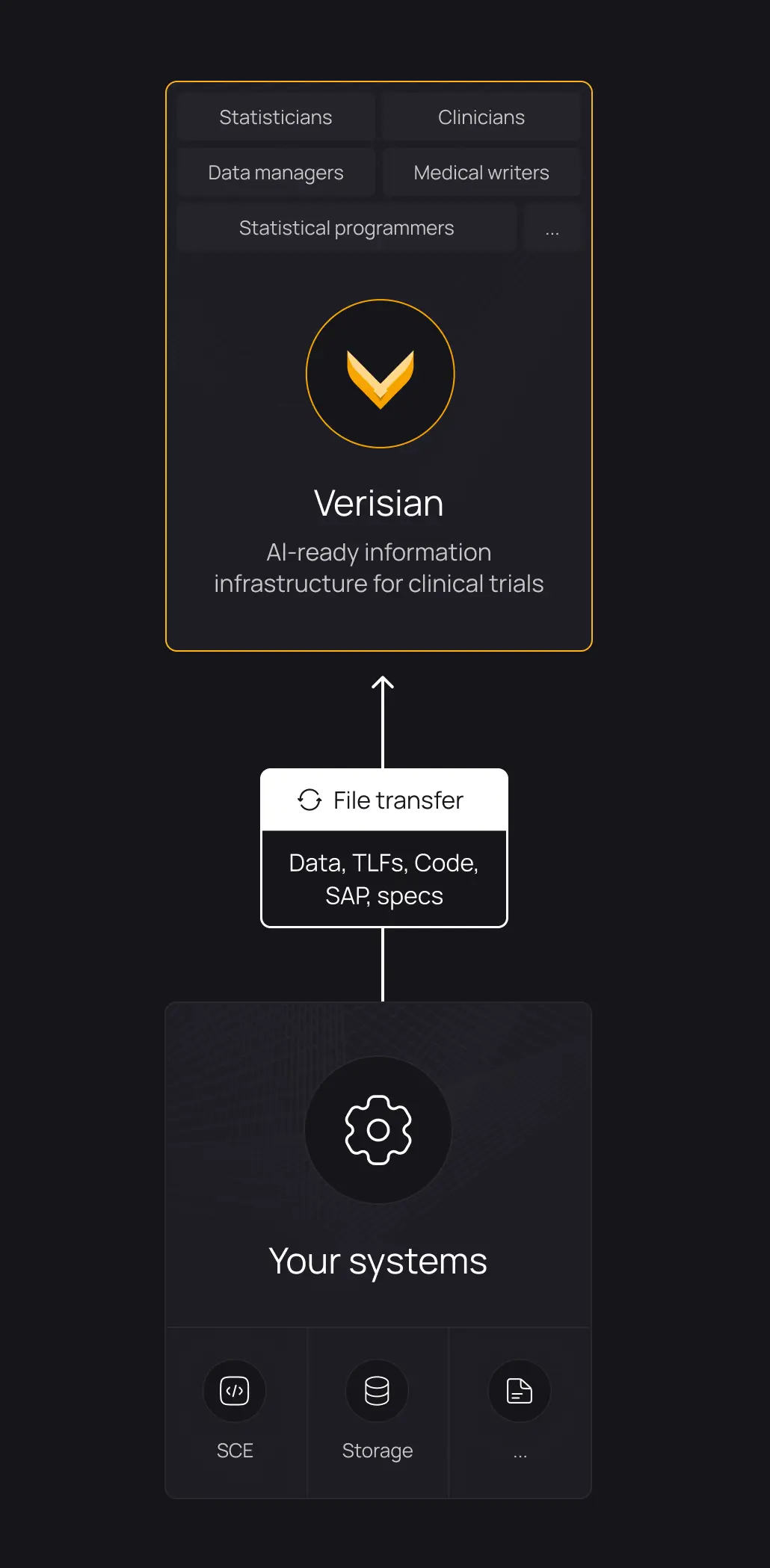
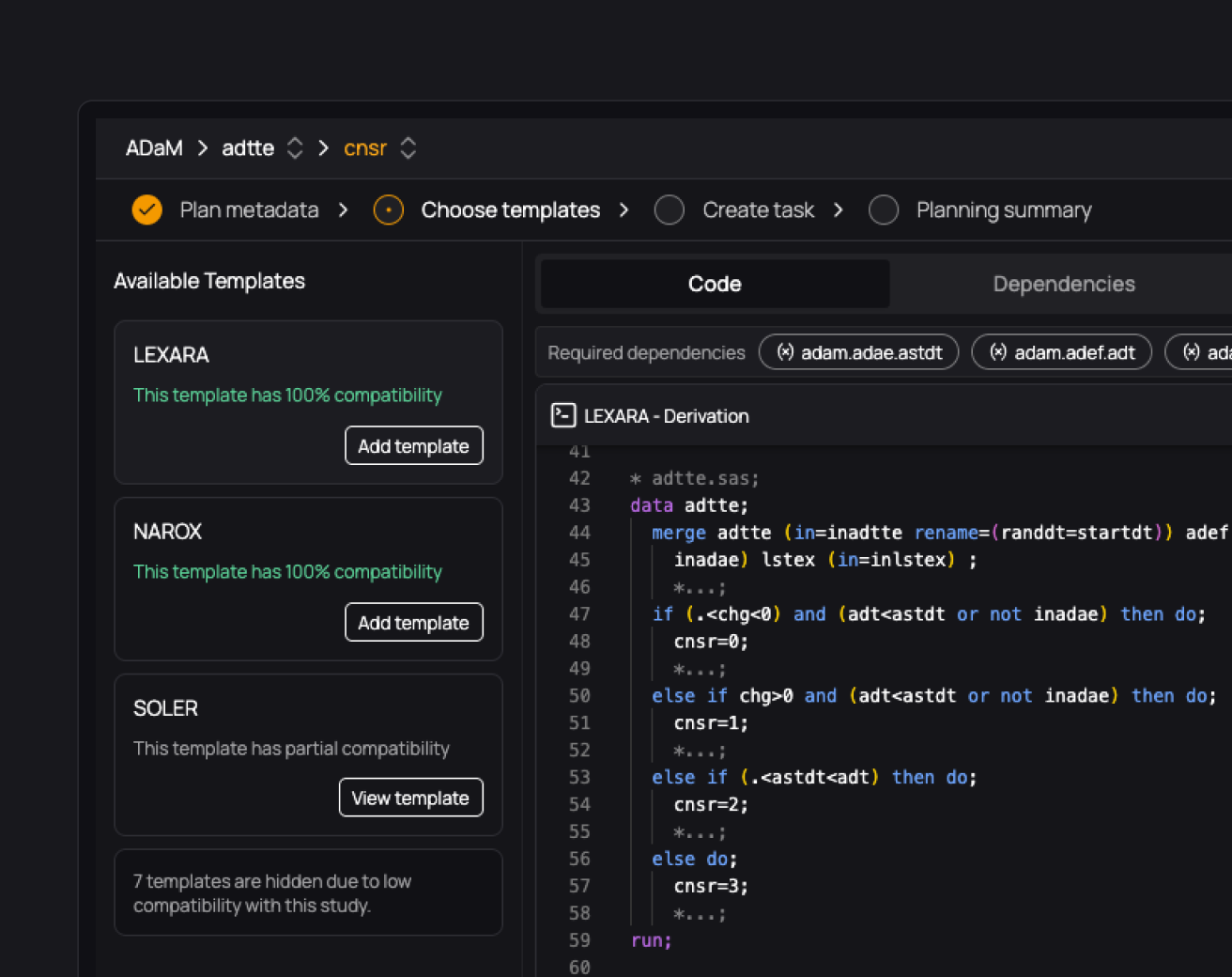
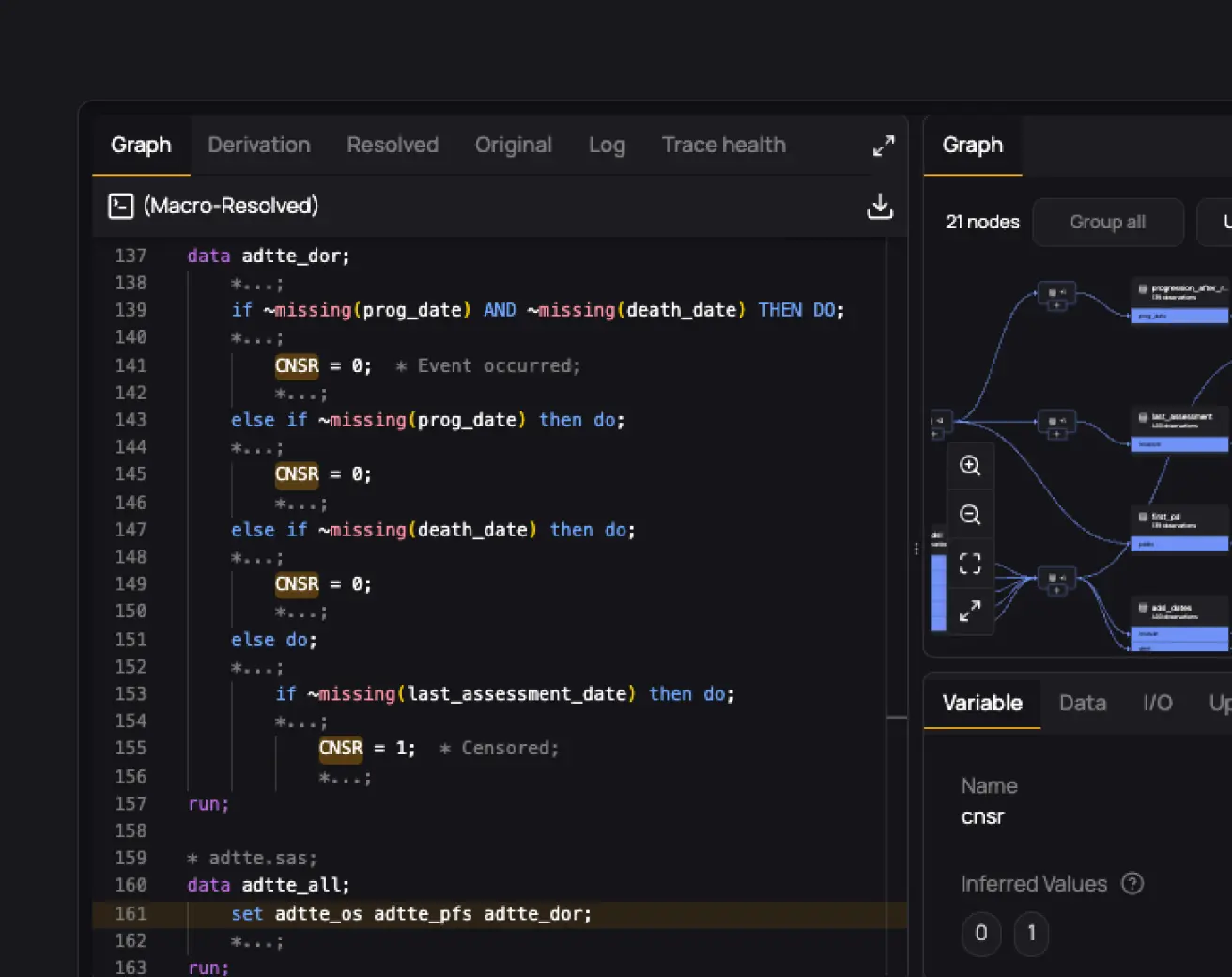
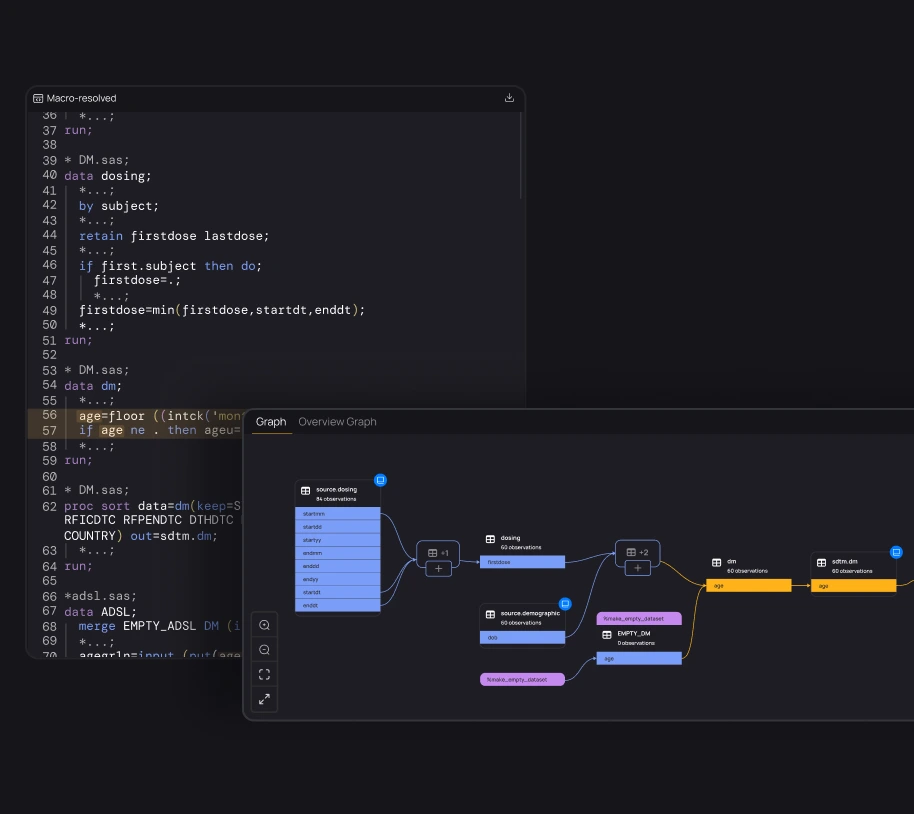
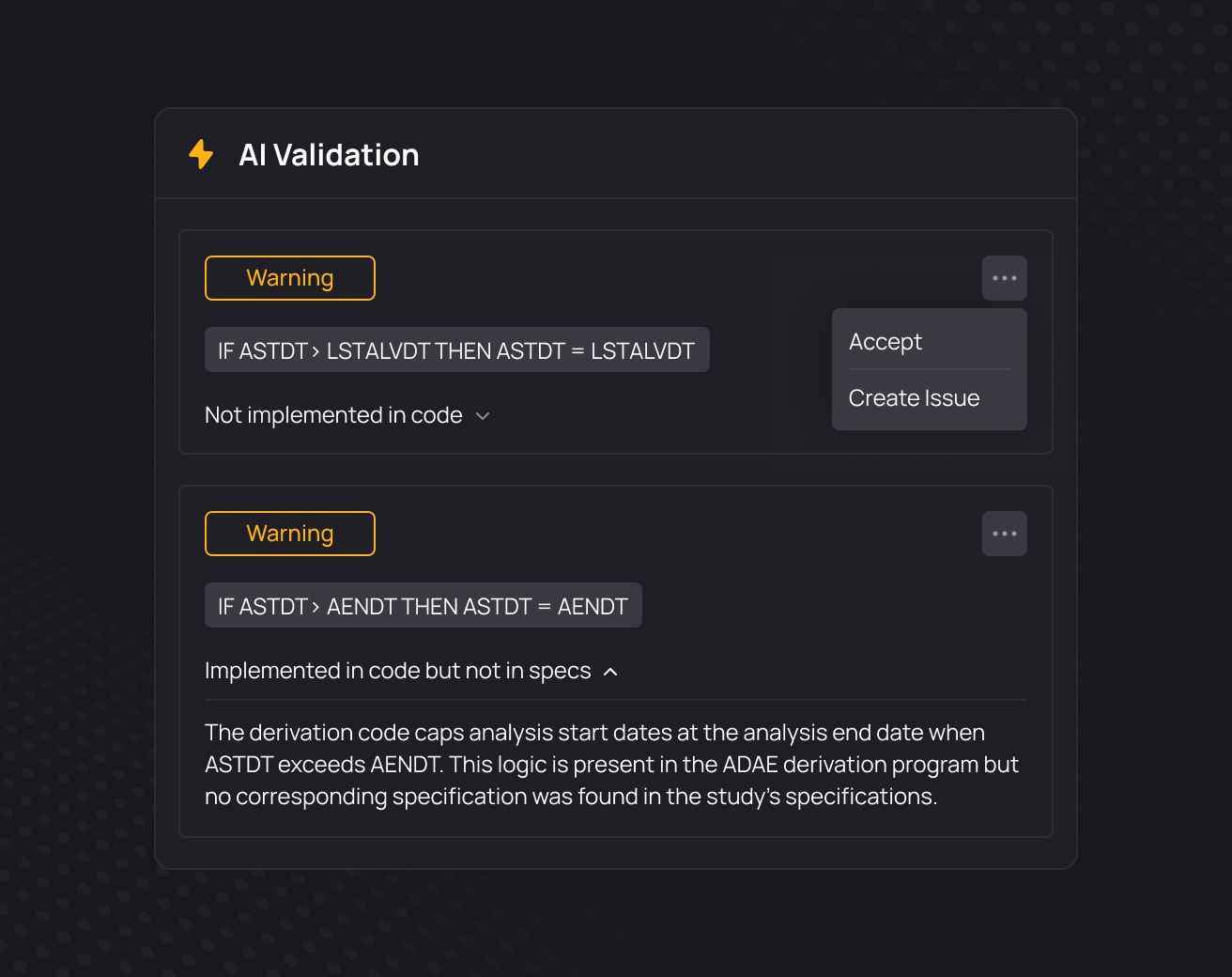
Onboard new programmers to studies 50-80% faster
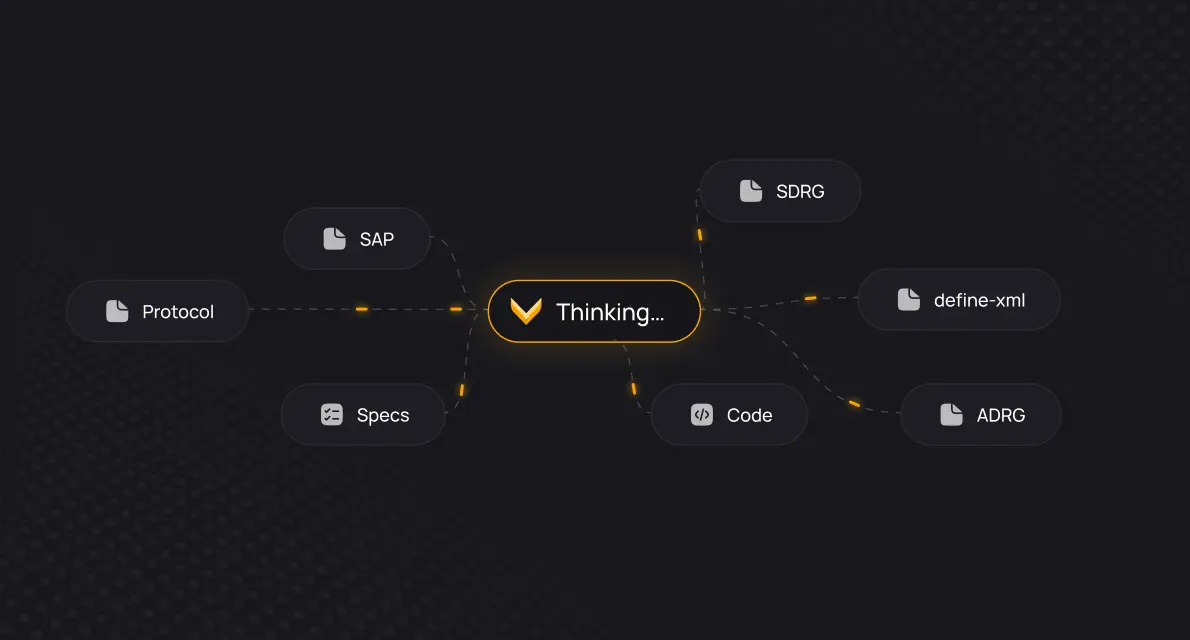
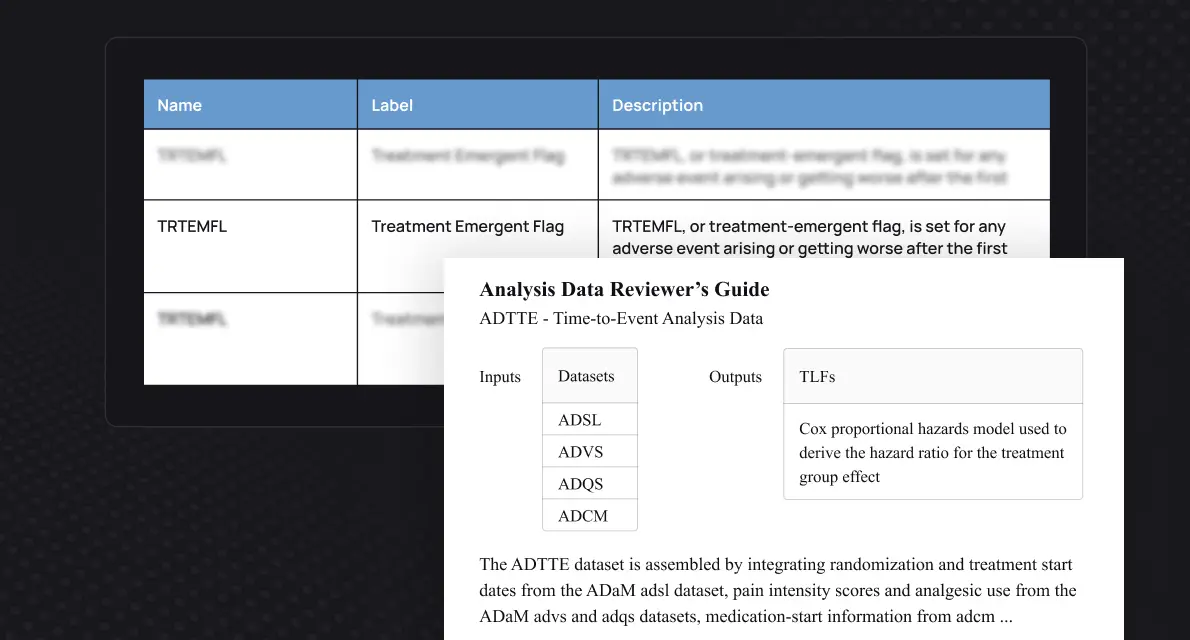
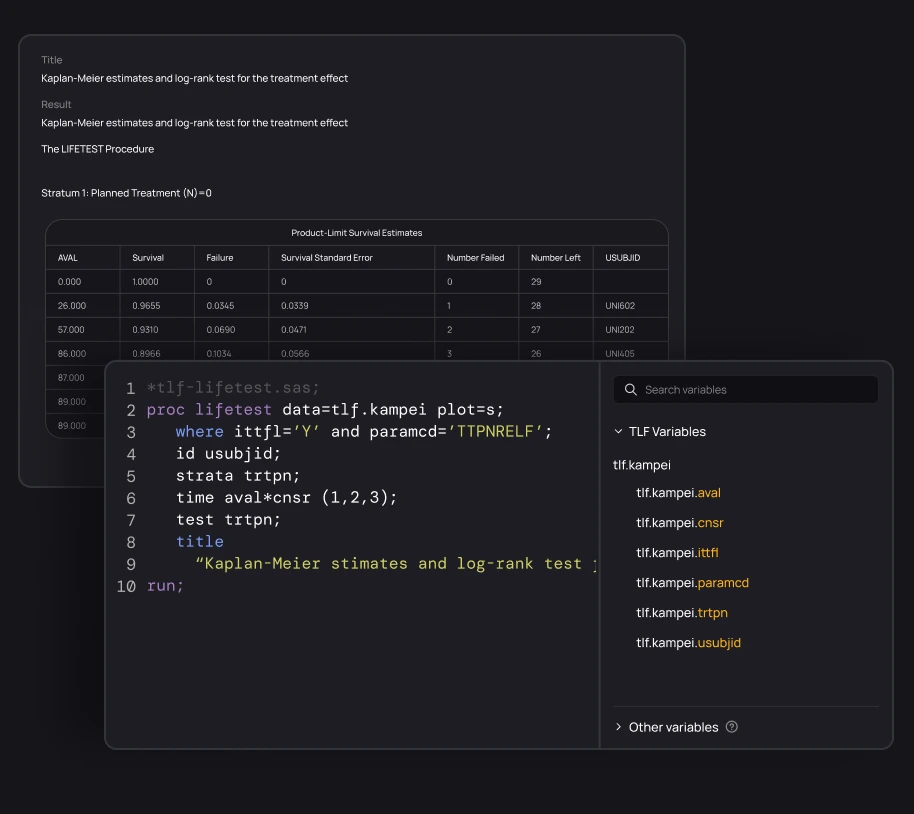
Give every programmer access to full study traceability. Explore and understand documentation, code, macros, variables, data, TLFs, and their relationships. With everything connected, onboarding to any study becomes effortless.